A device for diabetes management developed at the University could lead to a day when Type 1 diabetes patients no longer have to perform multiple blood tests a day or personally inject themselves with insulin. University researchers, in collaboration with Charlottesville company TypeZero Technologies, refined a software that automatically monitors blood glucose levels of Type 1 diabetes patients and determines how much insulin they should receive at a given moment.
According to researchers at the University, the artificial pancreas system may be a potential game changer for the over 1.25 million people suffering with Type 1 diabetes in the United States. The software aims to relieve patients of the stress and emotional burden that comes with constantly monitoring blood glucose levels and insulin intake.
Holland Edmonds is a Charlottesville native and Type 1 diabetes patient who tested the artificial pancreas system in clinical trials at the University’s Center for Diabetes Technology, CDT. The biggest impact for Edmonds was not having to monitor her blood glucose levels constantly.
“It was really nice to see even just for a few days what could possibly be on the market eventually, and to see that maybe I wouldn’t have to be worrying about it constantly,” Edmonds said. “[The artificial pancreas] would tell me if something was wrong and I didn’t actually have to do the worrying which was pretty amazing and it really excited me about what eventually could exist and could make my life a whole lot easier.”
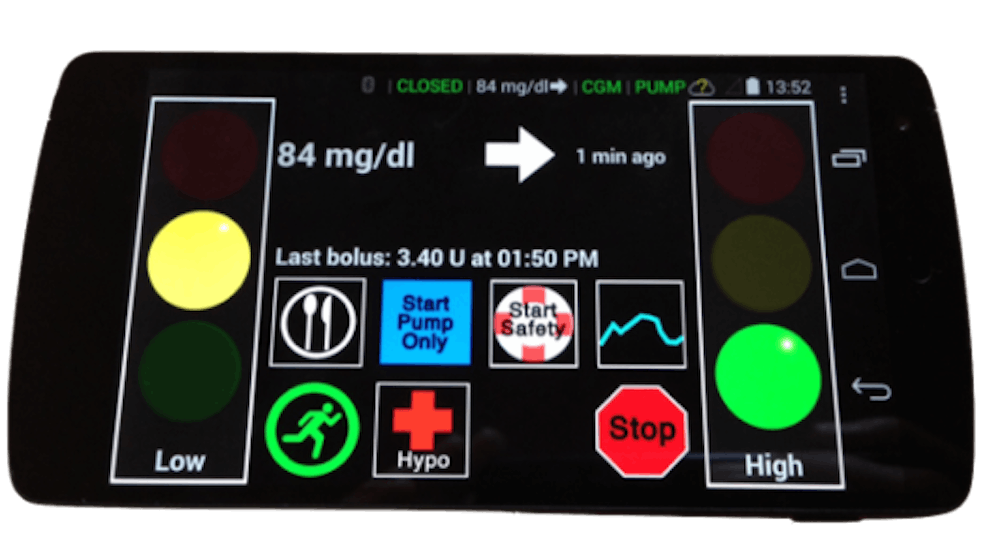
The artificial pancreas is a multi-device system that includes a continuous glucose monitoring — or CGM — sensor, an insulin pump and a control algorithm. The CGM sensor is implanted under the patient’s skin near the lower abdomen and continuously gathers information on their blood glucose levels. The information is sent to an external processor that contains a control algorithm that does a series of calculations to provide dosing instructions for the insulin pump. Based on these instructions, the insulin pump delivers insulin to the patient through a small flexible tube.
Currently, patients still utilize blood glucose devices, BGDs, to calibrate the CGM. However, researchers predict that as the technology for the artificial pancreas improves, these devices can be removed to eliminate as many patient decisions as possible.
Boris Kovatchev, a professor of psychiatry and neurobehavioral sciences in the School of Medicine, is the founding director at the CDT and the principal investigator in clinical trials for the artificial pancreas.
According to Kovatchev, patients that have the hardest time managing their diabetes are children. As such, the artificial pancreas can be especially groundbreaking for juveniles and their parents because it almost completely eliminates concern of fluctuations at night and allows kids to live more normal lives.
“For parents, the biggest fear and the biggest worry is that their kid will have low blood sugar overnight and that something bad is going to happen,” Kovatchev said. “This kind of system would take that fear away essentially. That is the first breakthrough of the system.”
The breakthrough is the control algorithm software that allows for communication between the CGM sensor and the insulin pump. Control algorithm software is able to read the CGM sensor’s information about the patient’s blood glucose levels and instruct the insulin pump to give the right amount of insulin more than 200 times a day. The algorithm can function on a computer, a smartphone or the insulin pump itself.
The artificial pancreas is often referred to as the “closed-loop system” or “automated insulin delivery system,” for its ability to enable constant communication between the CGM sensor and the insulin pump. That is to say, this software innovation could potentially create a the day when patients no longer have to prick their finger several times for blood sugar tests using a BGD.
Marc Breton, an associate professor of research at the CDT, is one of the principal investigators involved in the clinical trials of the artificial pancreas and is closely involved with the creation of system designs. Breton describes the computer algorithm technology as the missing piece that closes the loop between the patient’s CGM and the insulin pump through allowing constant communication between the two.
“What we do is we make the two talk to each other,” Breton said. “We put a brain in the middle, something that can analyze how much insulin has been administered, how much should be now, and do that every five minutes.”
Meagan Rollins is the senior operations manager for TypeZero Technologies Inc. The company is focused on development of an artificial pancreas application that lives on a commercial smartphone and talks to patients’ CGM.
According to Rollins, the artificial pancreas is monumental for Type 1 patients because of its ability to decrease risk of hypoglycemia, which allows them to live more comfortably and as close to their pre-diabetic lives as possible.
“It keeps patients out of hypoglycemia moreso than other systems that are currently on the market,” Rollins said. “Patients feel safe. Patients feel that this does reduce a lot of the minute by minute decisions they have to make. I do think our technology is a game changer.”
The average Type 1 patient will experience at least two episodes of hypoglycemia per week. Symptoms include decrease in energy, impaired cognition, mood swings and even death in severe episodes. To avoid hypoglycemia, individuals with Type 1 have to closely monitor what they eat, their activity and their insulin levels. The artificial pancreas system drastically decreases the worry Type 1 patients face of entering into hypoglycemia.
As for the future of diabetes management in the United States, Edmonds said that the constant glucose monitoring and automatic insulin delivery offered by artificial pancreas system provide the answers.
“Being able to not worry about it 24/7 was definitely the biggest change for me and it’s really helping a lot of other people with keeping them in range constantly,” Edmonds said. “It is an amazing thing that hopefully they’ll be able to perfect and put out on the market for all people with Type 1 diabetes.”
As of October 2018, the smartphone-based automated insulin delivery system has been used by a total of 425 subjects in clinical trials at the University. Currently, the artificial pancreas is not FDA approved, but it is in its last stage of clinical trials at the University and nine other site locations across the world, including Stanford University and Harvard University.