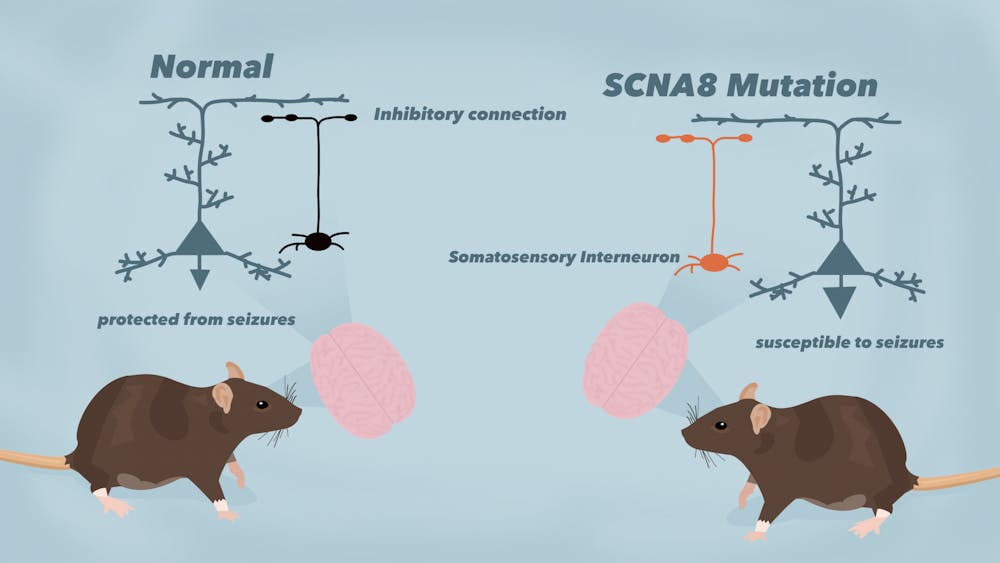
A study conducted by researchers in the School of Medicine found that mutations in the gene SCN8A led to epileptic seizures when expressed in brain cells called somatostatin interneurons. By identifying these cells as a factor in triggering seizures, this study provides a new way of thinking about how to treat a very severe form of epilepsy.
The research was published in the Journal of Neuroscience and focuses on SCN8A-related epilepsy with encephalopathy. Epilepsy is a spectrum of brain disorders that lead to seizures, which are surges of electrical activity in the brain.
Researchers first found the genetic mutation that causes SCN8A epilepsy in 2012, after young girl named Shay Emma Hammer had epilepsy and no one could detect the cause. Her father, geneticist Dr. Michael Hammer, set out to find what was causing his daughter’s disease, but Shay died when she was 15 years old. Her father’s team discovered the mutation in the SCN8A gene months after her death.
On average, patients with this disease have seizures beginning when they are just four months old and suffer from multiple types of seizures, movement disorders, intellectual disability and autistic-like features. According to the National Institute of Health, 10 percent of children diagnosed with this complication have died from sudden unexpected death in epilepsy.
Dr. Manoj Patel, associate professor in the School of Medicine’s Anesthesiology Department and principal investigator of the NIH grant that funded the research, keeps pictures of some of these children in his office.
“I meet the families and the children, and you get to know some of the children,” Patel said. “Some of these children are bedridden, they need to stay in their homes all the time. They can't move, they can't really do anything.”
Dr. Eric Wengert, who received his PhD from U.Va. in early 2021, ran the study and said that although this form of epilepsy is very rare, its severity makes a good model for trying to understand epilepsy more generally. Because researchers have pinpointed a genetic cause of the disease, they will be able to efficiently create models to study it.
“It can help us identify what really matters,” Wengert said.
The lab started working on SCN8A epilepsy a few years after the mutation was discovered in 2014. Researchers took the genetic mutation discovered in Shay and used sophisticated genetic tools to put that exact change into just the somatostatin interneurons of mice.
They observed whether the mice had seizures and observed brain slices from those who did to discover what happens on both the cellular and the behavioral levels because of that exact mutation.
Wengert, Patel and their team tried to trigger seizures in mice by exposing them to a very loud sound, which is known as an audiogenic seizure. They found that this SCN8A mutation in somatostatin interneurons alone was enough to make the mice have audiogenic seizures.
“We were shocked,” Patel said . “We didn't think it was going to work. We didn't think that that was going to be enough to make them have a seizure. But it was, and I realized then the [research] paper was going to be higher-impact.”
When studying seizures, most scientists focus on excitatory neurons, which are neurons that excite this activity. The results of this study were surprising because somatostatin interneurons are not excitatory neurons, but the opposite — inhibitory neurons.
“If epilepsy is a car that's going too fast, it could be because the throttle is too strong, or because the brakes are broken,” Wengert said. “We show that without changing the throttle at all, if you just mess up the brake, somehow the car gets going too fast, which is a little bit surprising in and of itself.”
In SCN8A epilepsy, these inhibitory neurons that are thought of as brakes are not only failing to brake, but can cause seizures by themselves — they can act as an accelerator.
Patel said this new research could change the way in which epilepsy is viewed since it shows that there are two things going on. Specifically, it has been shown that seizures are not caused only by the excitatory neurons or by “the accelerator.”
“This study is saying ‘don’t ignore the brake,’” Patel said.
The research also pinpoints a new place to look for ways to treat epilepsy.
“In the same way that you would fix a car differently that has broken brakes versus a broken throttle, you would design a treatment for epilepsy in the brain differently based on if it's a failure of inhibition mechanisms or if it's a failure of excitatory neurons,” Wengert said.
The accelerator is very difficult to control. The lab’s current project is instead trying to make the brake more effective to see if fixing the brake would be enough to stop the seizures.
Wengert added that developing a new treatment for SCN8A epilepsy will take a long time, but that is the vision for this work. Previous work from the lab is currently in clinical trials and Wengert and Patel hope the same will be true of this research in the future.
“We're a very rare lab. It's very focused on trying to actually bridge that gap between research and helping people,” Wengert said. “That's why we do what we do.”